Public Pool Regulation
The public pool regulation is governed by different standards. Here are some key reminders for good pool water quality management. Water quality in public pools must meet the required standards. Therefore, the following water testing points must be followed:
Regulation and Recommandation:
Here are the regulations for public pools, aquatic centers, semi-public pools and pools open to the public:
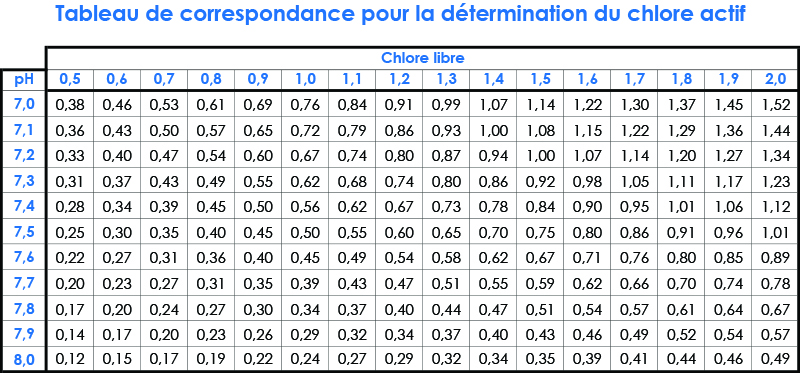
- For a pool WITHOUT stabilizer, active chlorine between 0.4 and 1.4 ppm
- For a pool WITH stabilizer, a free chlorine between 2 and 5 ppm
- Combined chlorine or also called chloramines below 0.6 ppm
- pH between 6.9 and 7.7
- A minimum of 30 liters of new water per bather per day
- An air level of trichloramine lower than 0.5 mg/m3 (for indoor pools)
- And the level of stabilizer (cyanuric acid) is less than 75 ppm
SYCLOPE also recommends monitoring the TAC and TH to verify the Taylor Balance.
- TAC (Total Alkalimetric Title) between 10 and 30°F
- TH (Hydrotimetric Title) between 10 and 20°F
You wish to obtain more information:
You can also consult the AFNOR,
or send us your requests for information by filling out the form below.

Water recycling time – What are the regulations for public pools?
Temps de recyclage de l’eau – Quelles réglementations en piscine publique ?
The water recycling time should be as follows (in accordance with current public pool regulations):
A. For pools that initially open before January 1, 2022:
- Eight hours (8h) for:
- a diving pool
- an underwater diving pit
- Thirty minutes (30 min) for a paddling pool
- One and a half hours (1h30) for other pools or parts of pools less than or equal to 1.5 m deep
- Four hours (4h) for other pools or parts of pools deeper than 1.5 m
- Thirty minutes (30 min) for whirlpools with a volume greater than or equal to 10m3 and fifteen minutes (15 min) for those with a volume less than 1m3
B. For all pools initially opened after January 1, 2022, or for pools initially opened prior to January 1, 2022, and undergoing renovation of water supply or discharge devices on or after that date:
- Eight hours (8h) for:
- a diving pool
- an underwater diving pit
- Thirty minutes (30 min)for:
- an individual, non-whirlpool pool
- a whirlpool with a volume of 10 m3 or more
- Fifteen minutes (15 min) for:
- a paddling pool
- a whirlpool with a volume of less than 10 m3
- One hour (1h) for slide receiving pools and slide arrival areas
- One and a half hours (1h30) for other pools or parts of pools with a depth of 1.50 m or less
- Four hours (4h)for other pools or parts of pools deeper than 1.50 m
For pools with a surface area of 100 m² or less:
- At least one surface skimmer is installed for 50 m² of water surface
For pools with a surface area of more than 100 square meters and less than or equal to 200 m² :
- At least one surface skimmer is installed for 50 m² of water surface, provided that an automatic regulation of disinfection and pH is set up;
- In the absence of the above-mentioned regulation for pools with a surface area of more than 100 m² and less than or equal to 200 m² or if it is a paddling pool or a whirlpool, at least one surface skimmer is installed for every 25 m² of water surface.
What is the definition of chorine stabilizer o isocyanuric acid?
Définition
Isocyanuric acid is the stabilizer of chlorine. It is used for outdoor pools to limit the impact of UV rays on the destruction of chlorine. Only isocyanuric acid is not a disinfectant, which is why it is added to the chlorinated product either in the form of pebbles or manually in operation.
The use of stabilizer implies a change of regulation: without stabilizer the regulation is based on active chlorine, with stabilizer it is based on free chlorine with a minimum of 2 ppm to be maintained.
It is recommended that the isocyanuric acid threshold be between 30 and 50 ppm, knowing that above 75 ppm chlorine becomes ineffective.

We are here to support you …
Do you have any questions? Need a diagnosis, recommendations? Need a documentation, an estimate ? The SYCLOPE team is at your disposal …