Active chlorine
How to maintain the correct level of active chlorine to ensure proper disinfection of water in public swimming pools? We help you to find solutions for your measurements.
What is active chlorine?
Also called hypochlorous acid (HOCl), it is the active and disinfectant form of chlorine, effective against microorganisms.
When added to potential chlorine or hypochlorite ion (OCl-), it forms free chlorine measured at DPD1.
Public swimming pool regulations require that the level of active chlorine be between 0.4 and 1.4 ppm.
How to determine the active Cl rate?
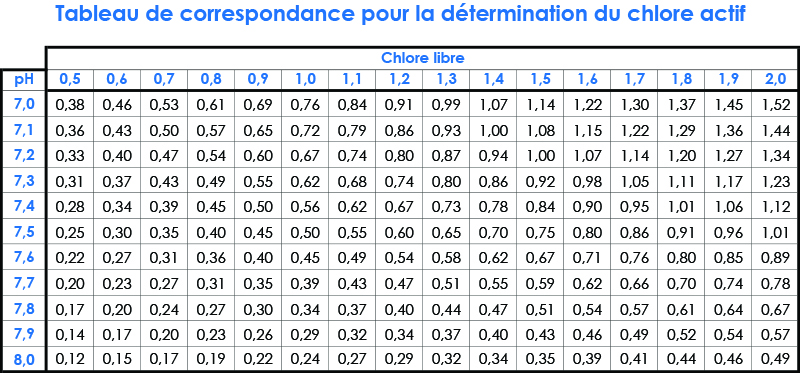
Il se détermine en fonction de la valeur de pH et de chlore libre (DPD1). La dissociation du chlore se calcule selon la courbe ci-dessous :
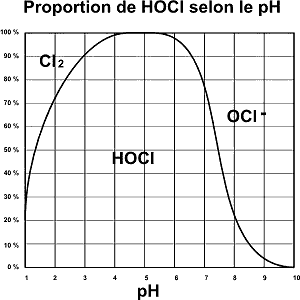
Example:
When measuring with a photometer, the following values are determined:
- 1 ppm free chlorine (DPD1) and pH at 7
= 70% HOCl, i.e. 0.7 ppm active chlorine and 0.3 ppm potential chlorine
Regulatory compliance - 1 ppm free chlorine (DPD1) and pH at 8
= 20% HOCl, i.e. 0.2 ppm active chlorine and 0.8 ppm potential chlorine
NOT in compliance with regulations (recommended level between 0.4 and 1.4 ppm)
It is therefore essential to maintain a pH between 7 and 7.4 in order to guarantee optimal disinfection of the water.
In order to facilitate the determination of the active Cl, the table below shows the rate as a function of pH.
How to measure Active Cl?
SYCLOPE proposes 2 solutions of determination:
Direct measurement of active Cl with amperometric probes
Combined with ALTICEO and ODITouch controllers, SYCLOPE amperometric probes allow to directly measure the active Cl level in water.
Rate calculation thanks to SYCLOPE controllers
The ALTICEO and ODITouch devices can determine the active Cl level by calculation: free chlorine depending on the pH value.
We are here to support you …
Do you have any questions? Need a diagnosis, recommendations? Need a documentation, an estimate ? The SYCLOPE team is at your disposal …